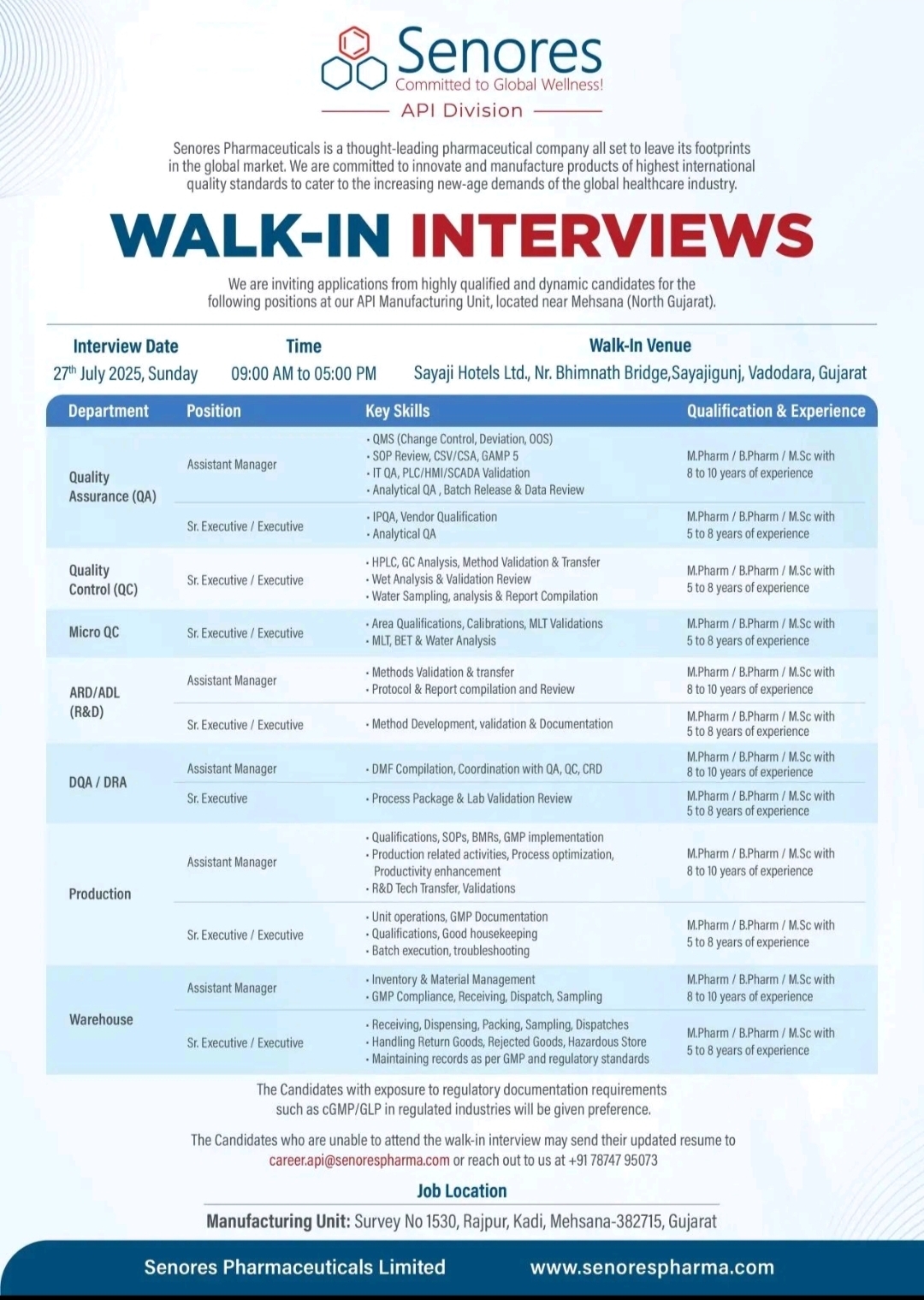
Exciting Career Opportunities at Acme Generics Pvt Ltd – Baddi, Himachal Pradesh
Are you an experienced pharmaceutical professional seeking a new and challenging opportunity? Acme Generics Pvt Ltd, a reputed name in the pharmaceutical manufacturing sector, is currently hiring for multiple roles across various departments at their Baddi, Himachal Pradesh facility. If you have experience in a USFDA-approved setup and are ready to take the next step in your career, this could be the perfect opportunity for you!
📌 Current Open Positions
🔍 Department: Quality Assurance
-
Designation: Senior Officer / Executive
-
Experience Required: 2 – 6 Years
🔍 Department: Quality Control
-
Designation: Executive / Sr. Executive
-
Experience Required: 5 – 10 Years
-
Specialization: Finished Goods (FG), Stability, QMS
🔍 Department: Production
-
Designation: Officer / Senior Officer / Operator
-
Experience Required: 2 – 10 Years
-
Specialization: Compression / Coating
✅ Eligibility Criteria
-
Candidates must have prior experience working in USFDA-approved pharmaceutical manufacturing facilities.
-
Strong knowledge and expertise in GMP, regulatory standards, and department-specific skills are essential.
📧 How to Apply
Interested candidates who meet the eligibility criteria are encouraged to send their updated CV to:
📩 Hr@acmegenerics.in
📱 Or connect via WhatsApp: +91 86289 68767
Why Join Acme Generics Pvt Ltd?
Acme Generics offers a dynamic work environment, excellent career growth opportunities, and a chance to be part of a dedicated team that’s committed to quality and compliance. Working at a USFDA-approved facility not only strengthens your industry credentials but also positions you for long-term growth in the global pharma industry.
If you're ready to grow your career with a trusted pharmaceutical manufacturer, don't miss this chance. Apply today and be a part of Acme Generics' journey of excellence.