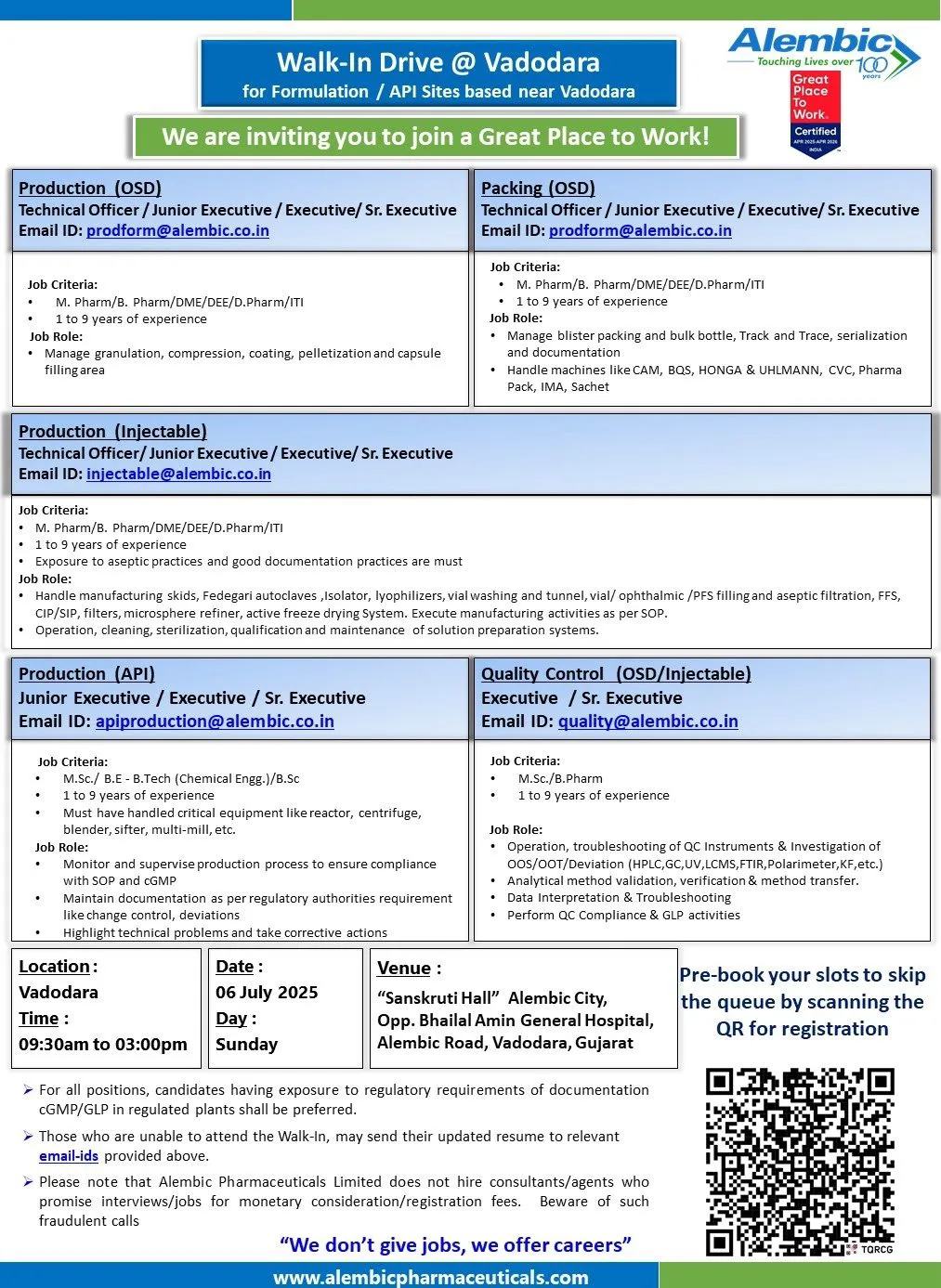
🚀 Elevate Your Pharma Career: Alembic Mega Walk-In Drive on July 6, 2025
Alembic Pharmaceuticals, a century-old leader in API and formulations manufacturing, is hosting a Mega Walk-In Drive on Sunday, 6th July 2025, near Vadodara. Highly recommended for professionals seeking roles in OSD, injectables, packing, production, or QC.
🏢 Why Alembic?
Alembic Pharmaceuticals Ltd., headquartered in Vadodara, ranks among India’s premier pharmaceutical firms. With a revenue of over ₹3,000 cr and global reach in areas like anti-infectives, cardiology, and neurology, the company is also Great Place to Work® certified and boasts USFDA-compliant manufacturing units.
Gujarat is the country’s top pharma hub—accounting for about 33% of national drug production and housing over 130 USFDA-certified facilities.
📅 Event Details
-
Date: Sunday, 6 July 2025
-
Time: 09:30 AM – 03:00 PM
-
Venue: Sanskruti Hall, Alembic City (opposite Bhailal Amin Hospital), Vadodara
💼 Available Roles & Requirements
1. Production – OSD
-
Responsibilities: Granulation, compression, coating, pelletization, capsule filling
-
Qualifications: M.Pharm / B.Pharm / D.Pharm / ITI
-
Experience: 1–9 years (prodform@alembic.co.in)
2. Packing – OSD
-
Tasks: Blister/bottle packing, serialization, machine operation (CAM, BQS, IMA, Uhlmann)
-
Qualifications: M.Pharm / B.Pharm / D.Pharm / ITI
-
Experience: 1–9 years (prodform@alembic.co.in)
3. Production – Injectables
-
Scope: Sterile ops, lyophilizers, FFS, CIP/SIP
-
Qualifications: M.Sc / B.Tech / B.Sc
-
Experience: Not specified (typically mid-range) (prodform@alembic.co.in)
4. Production – API
-
Focus: Reactor, centrifuge, multi-mill, documentation per cGMP
-
Qualifications: M.Sc / B.Tech (Chemical Engg.) / B.Sc
-
Experience: Not specified (prodform@alembic.co.in)
5. Quality Control – OSD/Injectable/API
-
Instruments: HPLC, GC, FTIR, UV, OOS/OOT
-
Qualifications: Relevant Chemistry/Pharma grads
-
Experience: Not specified
✅ Why You Should Attend
-
Direct hiring—no middlemen or fees
-
Align with GMP/GLP norms in regulated environments
-
Work with legacy brand in a globally scaled setting
-
Comprehensive opportunity across various technical disciplines
📝 Interview Preparation Guide
-
Pre-register via QR link to avoid wait
-
Document Checklist: resume, educational/experience certificates, passport photos, ID proof
-
Study Up: Brush up on sterile production, API workflows, packaging, analytical instrumentation, and cGMP processes
-
Arrive Early: Beat the crowd and secure your slot
🌐 Industry Snapshot – Gujarat
Vadodara–Ahmedabad region is India’s pharmaceutical powerhouse—hosting top names like Alembic, Zydus, Cadila, and Sun Pharma. It leads the nation in production, exports, and regulatory standards, offering stable and high-exposure career paths in manufacturing and quality disciplines.
✅ Final Thoughts
Alembic’s walk-in drive on 6 July 2025 provides a well-rounded opportunity for pharma professionals in OSD, injectables, API, packing, or QC. Whether you're a fresh graduate or seasoned technician, this is a prime chance to join a leading pharma entity with growth potential and robust systems.